老化或衰老(参见拼写差异)是变老的过程。该术语特别指人类,许多动物和真菌,而例如细菌,多年生植物和一些简单动物可能是长寿的。从广义上讲,衰老可以指生物体内已停止分裂(细胞衰老)或物种种群(种群老化)的单细胞。
在人类中,衰老代表了人类随时间变化的积累,[1]包括身体,心理和社会变化。例如,反应时间可能随着年龄的增长而减慢,而世界事件和智慧的知识可能会扩大。老龄化是大多数人类疾病最着名的风险因素之一:[2]全球每天死亡的大约150,000人中,约三分之二死于与年龄相关的原因。
衰老的原因尚不确定;目前的理论被赋予损害概念,其中损害的累积(例如DNA氧化)可能导致生物系统失效,或者导致程序化的老化概念,由此内部过程(例如DNA甲基化)可能导致老化。程序化衰老不应与程序性细胞死亡(细胞凋亡)相混淆。
1934年发现,卡路里限制可以延长大鼠寿命50%,这推动了延迟和预防衰老的研究。
视频: ↓ 为什么我们的身体会老化?
https://cache.tv.qq.com/qqplayerout.swf?vid=o0751f5dxbt
内容
1 老龄化与长寿
2 衰老的影响
3 生物学基础
3.1 程序化因素
3.2 与损害有关的因素
4 预防和延误
4.1 生活
4.2 医疗干预
4.3 研究项目和奖项
5 社会与文化
5.1 经济学
5.2 社会学
5.3 医疗保健需求
5.4 对衰老的自我认知
5.5 成功老化
5.6 文化参考
6 参考
衰老与长寿
Immortal Hydra, a relative of the jellyfish

长寿的水,,海蜇的亲戚
人类和其他物种的成员,特别是动物,必然会经历衰老​​和死亡。真菌也可以衰老。[3]相比之下,许多物种可以被认为是永生的:例如,细菌裂变产生子细胞,草莓植物生长跑步者以产生自身的克隆,而Hydra属中的动物具有再生能力,通过它们可以避免老年人死亡。
地球上的早期生命形式,至少从37亿年前开始,[4]是单细胞生物。这些生物(原核生物,原生动物,藻类)通过裂变繁殖成子细胞;因此,不要衰老,天生长寿。[5] [6]
随着有性生殖的进化,随着大约10亿年前真菌/动物王国的出现,以及3.2亿年前种子生产植物的进化,个体生物的衰老和死亡成为可能。此后,有性生物可以传递一些遗传物质来产生新的个体,并且就其物种的生存而言,它本身就可以成为一次性的。[7]然而,这种经典的生物学观点最近被大肠杆菌细菌分裂成可区分的子细胞的发现所扰乱,这开启了细菌中“年龄等级”的理论可能性。[8]
即使在人类和其他致命物种中,也存在具有永生潜力的细胞:癌细胞在维持细胞培养物(如HeLa细胞系)[9]和特定干细胞如生殖细胞时丧失了死亡能力。 (产生卵子和精子)。[10]在人工克隆中,成体细胞可以恢复到胚胎状态,然后用于生长新的组织或动物而不会老化。[11]然而,正常的人类细胞在实验室培养中约50次细胞分裂后死亡(Hayflick Limit,由Leonard Hayflick于1961年发现)。[9]
最近的证据表明105岁以后与年龄相关的死亡风险高原。[12]
衰老的影响
Enlarged ears and noses of old humans are sometimes blamed on continual cartilage growth, but the ca ...

老人的耳朵和鼻子的扩大有时被归咎于持续的软骨生长,但原因更可能是引力。[13]
Age dynamics of the body mass (1, 2) and mass normalized to height (3, 4) of men (1, 3) and women (2 ...
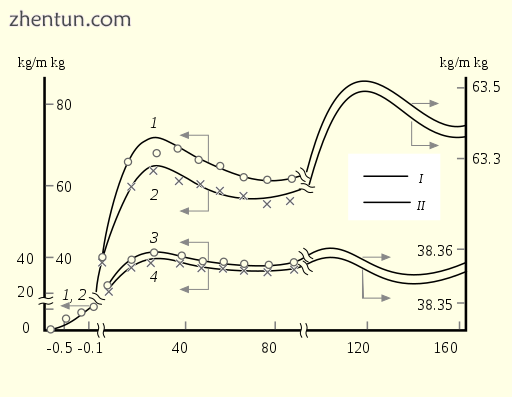
体重(1,2)的年龄动态和男性(1,3)和女性(2,4)的身高(3,4)标准化。[14]
Comparison of a normal aged brain (left) and a brain affected by Alzheimer's disease (right).
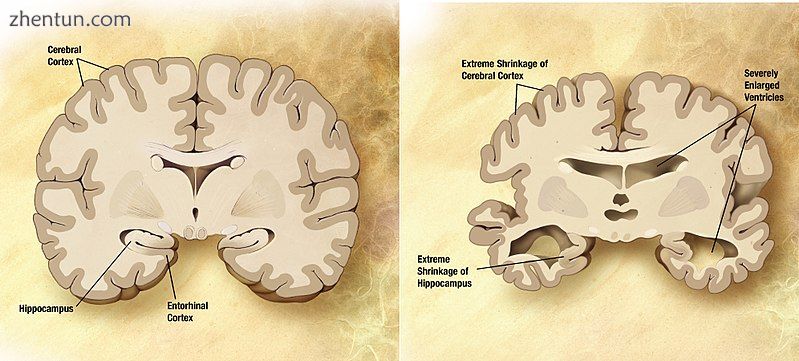
比较正常老年大脑(左)和受阿尔茨海默病影响的大脑(右)。
另见:老年人§老年人的标记
大多数人或大部分人在其一生中经历了许多特征性衰老症状。
青少年失去了幼儿听到20 kHz以上高频声音的能力。[15]
在20年代中期,认知开始下降。[16] [17]
皱纹的发展主要是由于光老化,特别是影响阳光照射的区域(面部)。[18]
在20年代中期达到顶峰后,女性生育率下降。[19]
在30岁之后,人体的质量减少到70年,然后显示阻尼振荡。[14]
35岁以上的人有发展老花眼的风险。[20] [21]大多数人在45-50岁时从老花镜中受益。[22]原因是通过降低α-晶状体蛋白的水平使晶状体硬化,这一过程可能会被更高的温度加速。[22] [23]
50岁左右,头发会变灰。[24] 50岁以下的模式脱发影响约30%-50%的男性[25]和四分之一的女性[26]。
更年期通常发生在49至52岁之间。[27]
在60-64岁年龄组中,骨关节炎的发病率上升至53%。然而,只有20%的人报告在这个年龄段导致骨关节炎。[28]
几乎一半的75岁以上的人有听力损失(老年性耳聋)抑制口语交流。[29]许多脊椎动物如鱼类,鸟类和两栖动物在老年时不患老年性耳聋,因为它们能够再生其耳蜗感觉细胞,而包括人类在内的哺乳动物遗传性地丧失了这种能力。[30]
到80岁时,超过一半的美国人患有白内障或进行过白内障手术。[31]
脆弱,定义为肌肉质量和活动性的丧失,影响超过85岁的人的25%。[32] [33]
动脉粥样硬化被归类为衰老疾病。[34]它导致心血管疾病(例如中风和心脏病)[35],这是全球最常见的死亡原因。[36]
人类的最大寿命建议为“在可预见的未来”115年。[37] [38]最古老可靠记录的人是Jeanne Calment,他获得了122年并于1997年去世。
随着年龄的增长,痴呆症变得更加普遍。[39]大约有3%的人年龄在65到74岁之间,19%在75到84岁之间,而将近一半的85岁以上的人患有痴呆症。[40]范围从轻度认知障碍到阿尔茨海默病,脑血管疾病,帕金森病和Lou Gehrig病的神经退行性疾病。此外,许多类型的记忆随着衰老而下降,但不是语义记忆或词汇定义等一般知识,这通常会增加或保持稳定直到成年后期[41](参见老龄化大脑)。情报随着年龄的增长而下降,尽管速度根据类型而有所不同,事实上可能在整个生命周期中保持稳定,只有在人们接近生命结束时突然下降。因此,认知能力下降率的个体差异可以用具有不同生命长度的人来解释。[42]大脑有变化:20岁以后,大脑有髓鞘轴突的总长度每十年减少10%。[43] [44]
年龄可能导致视力障碍,从而减少非语言交流,[45]这可能导致孤立和可能的抑郁。黄斑变性导致视力丧失并随年龄增长而增加,影响了80岁以上人群的近12%。[46]这种退化是由废物循环的系统性变化和视网膜周围异常血管的生长引起的。[47]
可以区分“近端衰老”(由于近期因素引起的基于年龄的影响)和“远端衰老”(基于年龄的差异,可以追溯到人类早年的原因,如 儿童脊髓灰质炎)。[42]
衰老是大多数人类疾病最着名的危险因素之一。[2] 在全球每天死亡的大约150,000人中,每天约有三分之二 - 100,000人死于与年龄相关的原因。 在工业化国家,这一比例更高,达到90%。[48] [49] [50]
生物学基础
主要文章:衰老
95-year-old woman holding a five-month-old boy

95岁的女子抱着一个五个月大的男孩
目前,研究人员才刚刚开始了解衰老的生物学基础,即使在相对简单和短命的生物体如酵母中也是如此。[51]人们对哺乳动物的衰老知之甚少,部分原因是即使是小型哺乳动物(如小鼠)的寿命也要长得多(约3年)。用于研究衰老的模式生物是线虫秀丽隐杆线虫,由于其寿命短至2-3周,我们能够轻易地进行遗传操作或通过RNA干扰或其他因素抑制基因活动。[52]大多数已知的突变和延长寿命的RNA干扰目标首先在秀丽隐杆线虫中发现[53]。
提出影响生物老化的因素[54]分为两大类,即程序化和损害相关的。程序化因素遵循生物学时间表,也许可能是监管儿童生长发育的时间表的延续。该规定将取决于影响负责维持,修复和防御反应的系统的基因表达的变化。与损害有关的因素包括对生物体的内部和环境攻击,这些生物体在不同程度上造成累积损害。[55]
在一篇详细的综述中,Lopez-Otin及其同事(2013年)通过损伤理论的视角讨论衰老问题,提出了各种生物体特别是哺乳动物衰老的九个代谢“标志”:[56]
基因组不稳定性(核DNA,mtDNA和核层中积累的突变)
端粒消耗(作者注意到人工端粒酶赋予其他致死细胞非癌症永生物)
表观遗传改变(包括DNA甲基化模式,组蛋白的翻译后修饰和染色质重塑)
蛋白质稳态丧失(蛋白质折叠和蛋白水解)
解除管理的营养传感(与生长激素/胰岛素样生长因子1信号通路有关,这是进化过程中最保守的老化控制途径,其目标是FOXO3 / Sirtuin转录因子和mTOR复合物,可能对热量有反应限制)
线粒体功能障碍(作者指出,最近的研究不再支持老化和活性氧物质线粒体生成增加之间的因果关系)
细胞衰老(在某些组织中不再分裂细胞的累积,特别是由p16INK4a / Rb和p19ARF / p53诱导阻止癌细胞增殖的过程)
干细胞衰竭(在作者看来由上面列出的损伤因素引起)
改变的细胞间通讯(尤其包括炎症,但也可能包括其他细胞间相互作用)
有三种主要的代谢途径可以影响衰老速度,讨论如下:
FOXO3 / Sirtuin途径,可能对热量限制有反应
生长激素/胰岛素样生长因子1信号通路
叶绿体中线粒体[57]和(植物中)电子传递链的活性水平。
这些途径中的大多数可能会分别影响衰老,因为同时瞄准它们会导致寿命增加。[58]
程序化因素
不同物种的老化速率差异很大,这在很大程度上是基于遗传的。例如,从草莓,马铃薯到柳树的许多多年生植物通常通过营养繁殖产生自身的克隆,因此可能是长寿的,而诸如小麦和西瓜的一年生植物每年死亡并通过有性繁殖繁殖。 2008年,人们发现一年生植物拟南芥中仅有两个基因失活,导致其转变为潜在的长寿多年生植物。[59]到目前为止,已知最古老的动物是15,000年前的南极海绵[60],可以在性和克隆方面进行繁殖。
除了克隆的长寿之外,还有某些物种的个体寿命在地球的生命形态中脱颖而出,包括5062年[61]或5067年的无脊椎动物,[60]无脊椎动物如硬蛤(在新英格兰称为quahog) 508年,[62]格陵兰鲨鱼400年,[63]各种深海管蠕虫超过300年,[64]鱼类像鲟鱼和石斑鱼,海葵[65]和龙虾。[66] ] [67]据说这些生物体的衰老可以忽略不计。[68]遗传方面也已在人类百岁老人的研究中得到证实。
在实验室环境中,研究人员已经证明,特定基因的选择性改变可以在酵母和蛔虫中大大延长寿命,在果蝇中则更少,而在小鼠中则更少。一些靶向基因具有跨物种的同源物,并且在某些情况下与人的长寿有关。[69]
DNA甲基化:自20世纪60年代后期以来,人们就已经知道年龄对DNA甲基化水平的强烈影响。[70] Horvath假设DNA甲基化年龄测量表观遗传维持系统的累积效应,但细节未知。血液的DNA甲基化年龄可预测晚年的全因死亡率[71] [72] [73]此外,过早老化的小鼠可以通过部分“重置”其细胞中的甲基化模式而恢复活力并且它们的寿命延长30%(完全重置导致不期望的永生癌细胞)。通过激活四种Yamanaka DNA转录因子--Sox2,Oct4,Klf4和c-Myc(之前已经常规用于从克隆的成年皮肤细胞产生幼小动物)实验性地实现了重置为幼年状态。[74] [75] ]
端粒:在人类和其他动物中,细胞衰老归因于每个细胞分裂时端粒的缩短; [76]当端粒变得太短时,细胞衰老并死亡或停止繁殖。[77]因此,端粒的长度是Hayflick预测的“分子钟”。[78] [79]然而,野生小鼠品系中的端粒长度与寿命无关[80],缺乏酶端粒酶的小鼠的寿命没有显著缩短。[81]实验室老鼠的端粒比人类端粒长很多倍。[82]另一个警告是,对近1000名人类进行了为期十年的研究表明,虽然有些人确实会随着时间的推移缩短端粒,但有三分之一的人没有。[83]
FOXO3A基因的变异对人类的预期寿命产生了积极的影响,并且在生活到100岁以上的人群中更常见 - 而且,这在世界范围内似乎是真实的。[84] [85] FOXO3A作用于sirtuin基因家族,这些基因对酵母和线虫的寿命也有显著影响。 Sirtuin反过来抑制mTOR。[86]
热量限制导致各种物种的寿命延长,这种影响尚不清楚[58],但可能是由mTOR途径的营养传感功能介导的。[87]
mTOR是一种抑制自噬的蛋白质,它通过胰岛素信号通路与衰老有关。 mTOR通过营养和生长线索发挥作用,使科学家们相信饮食限制和mTOR与长寿有关。当生物限制其饮食时,mTOR活性降低,这允许自噬水平增加。这可以回收旧的或受损的细胞部分,从而延长寿命并减少肥胖的机会。这被认为可以防止血液中葡萄糖浓度的峰值,从而导致胰岛素信号传导减少。这与mTOR活化较少有关。因此,长寿与热量限制和抑制mTOR的胰岛素敏感性有关,这反过来允许自噬更频繁地发生。 mTOR抑制和自噬可能会减少活性氧对身体的影响,从而损害DNA和其他有机物质,从而延长寿命。[88]为支持这一论点,观察到一些据称的抗衰老疗法包括雷帕霉素,二甲双胍,小檗碱,2-脱氧葡萄糖,维生素D3,阿司匹林和白藜芦醇被证明可抑制mTOR信号传导并同时降低氧化的组成水平。
An elderly Somali woman

一位索马里老人
能量产生和消耗(能量稳态)之间的细胞平衡需要在衰老期间进行严格的调节。 2011年,研究表明AMP活化蛋白激酶的乙酰化水平随着酵母的年龄而变化,并且防止这种变化会减缓酵母的衰老。[98]
与损害有关的因素
衰老的DNA损伤理论:DNA损伤被认为是癌症和衰老的共同基础,并且有人认为DNA损伤的内在原因是衰老的最重要驱动因素。[99] [100] [101]遗传损伤(DNA的异常结构改变),突变(DNA序列的变化)和表观突变(基因启动子区域的甲基化或调节基因表达的DNA支架的改变)可导致基因表达异常。 DNA损伤导致细胞停止分裂或诱导细胞凋亡,通常影响干细胞池并因此阻碍再生。然而,对小鼠的终身研究表明,大多数突变发生在胚胎和儿童发育期间,当细胞经常分裂时,因为每次细胞分裂都是DNA复制错误的机会。[102]
遗传不稳定性:在心肌细胞中,狗每年在心肌细胞中损失约3.3%的DNA,而人类每年损失约0.6%的心肌DNA。这些数字接近两个物种的最大寿命比率(120年与20年,比率为6/1)。狗和人之间的比较百分比在脑和淋巴细胞中每年DNA损失也相似。正如第一作者Bernard L. Strehler所说,“......遗传损伤(特别是基因丢失)几乎肯定(或可能是)衰老的主要原因。”[103]
积累废物:
细胞中废物的积累可能会干扰新陈代谢。例如,称为脂褐素的废物是通过将脂肪与蛋白质结合的细胞中的复杂反应形成的。这种废物在细胞中以小颗粒的形式积聚,随着年龄的增长,这种颗粒会逐渐增大。[104]
老化酵母细胞的标志似乎是某些蛋白质的过量产生。[51]
自噬诱导可以增强与神经退行性疾病相关的有毒细胞内废物的清除,并且已经被全面证明可以改善酵母,蠕虫,苍蝇,啮齿动物和灵长类动物的寿命。然而,鉴于在老化过程中也可能发生自噬上调,这种情况变得复杂。[105]通过热量限制,运动和低脂饮食增强肥胖小鼠的自噬(但在这些小鼠中显然与AMP激活的蛋白激酶的激活无关,见上文)。[106]
磨损理论:与衰老相关的变化这一非常普遍的观点是随着时间的推移而累积的偶然伤害的结果。[107]
积累错误:衰老是由逃避证据阅读机制的偶然事件引起的,这种机制会逐渐破坏遗传密码。
交联:衰老是由于交联化合物的积累导致干扰正常细胞功能的观点。[79] [108]
对mtDNA mutator小鼠的研究表明,体细胞mtDNA突变水平的增加直接导致各种衰老表型。作者提出,mtDNA突变导致呼吸链缺陷细胞,从而导致细胞凋亡和细胞丢失。然而,他们在实验上产生了疑问,这是因为线粒体突变和功能障碍导致活性氧(ROS)的产生增加。[109]
自由基理论:自由基或更常见的活性氧或氧化应激造成的损害会造成可能导致我们认为是衰老症状的损害。[79] [110] Michael Ristow的研究小组提供的证据表明,卡路里限制的影响可能是由于线粒体内自由基的形成增加,导致抗氧化防御能力增强的二次诱导。[111]
DNA氧化和热量限制:热量限制可减少衰老大鼠和小鼠器官中8-OH-dG DNA的损伤。[112] [113]因此,氧化性DNA损伤的减少与较慢的衰老速度和较长的寿命有关。[114]
预防和延误
另见:寿命延长
生活方式
热量限制严重影响许多动物的寿命,包括延迟或预防许多与年龄有关的疾病的能力。[115]通常情况下,这涉及热量摄入量为自由采食动物消耗量的60-70%,同时仍保持适当的营养摄入量。[115]在啮齿动物中,已经证明这可以使寿命延长50%; [116]酵母和果蝇也会出现类似的效应。[115]在卡路里限制饮食中,人类没有寿命数据,[90]但有几份报告支持保护免受与年龄有关的疾病。[117] [118]关于恒河猴的两项正在进行的主要研究最初揭示了不同的结果;威斯康星大学的一项研究表明,热量限制确实延长了寿命,[119]国家老龄化研究所(NIA)的第二项研究发现,没有热量限制对长寿的影响。[120]然而,这两项研究都显示出许多健康参数的改善。尽管卡路里摄入量相似,但两种研究的饮食成分不同(特别是威斯康星州研究中的高蔗糖含量),猴子的起源不同(印度,中国),最初表明遗传和膳食成分,而不仅仅是减少卡路里,是长寿的因素。[90]然而,在2014年的一项比较分析中,威斯康星州的研究人员发现,与其他猴子种群相比,据称非饥饿的NIA对照猴实际上体重不足,并认为这是由于NIA的分配饲喂方案与威斯康星州真正相比不受限制的自由采食协议。[121]他们的结论是,适度的卡路里限制而不是极端的卡路里限制足以在研究的恒河猴中产生观察到的健康和长寿益处。[122]
Hayflick在他的“How and Why We Age”一书中说,卡路里限制可能对人类无效,引用了巴尔的摩老年人纵向研究的数据,该数据表明,瘦身不利于长寿。[需要引用验证] [123]同样有时人们声称,晚年适度肥胖可以提高生存率,但是较新的研究已经发现了混杂的因素,如终末期疾病导致的体重减轻。一旦考虑了这些因素,65岁以上的最佳体重对应于较瘦的体重指数23至27。[124]
另外,饮食限制的好处也可以通过改变宏观营养成分来减少蛋白质摄入量而不改变卡路里水平,从而导致类似的寿命延长。[125] [126]膳食蛋白质限制不仅抑制mTOR活性,而且抑制IGF-1,这两种机制与衰老有关。[87]具体而言,减少亮氨酸摄入量足以抑制mTOR活性,可通过减少动物食物消耗来实现。[127] [128]
地中海饮食被认为可以降低患心脏病和早逝的风险。[129] [130]降低死亡率风险的主要因素似乎是蔬菜,鱼类,水果,坚果和单不饱和脂肪酸(即橄榄油)的消费量较高。[131]
睡眠量对死亡率有影响。生活时间最长的人每晚睡6到7个小时。[132] [133]睡眠不足(<5小时)使心血管疾病死亡的风险增加一倍以上,但过多的睡眠(> 9小时)与死亡风险加倍有关,但主要不是心血管疾病。[134]每天睡眠超过7至8小时一直与死亡率增加有关,尽管其原因可能是抑郁症和社会经济状况等其他因素,这些因素在统计学上是相关的。[135]来自非洲和南美洲的狩猎 - 采集部落的睡眠监测显示,各大洲的睡眠模式相似:平均睡眠时间为6.4小时(夏季/冬季相差1小时),下午小睡(午睡)不常见,失眠非常罕见(比工业社会少十倍)。[136]
体育锻炼可能会延长预期寿命。[137]与没有身体活动的人相比,参加中度至高度体育锻炼的人死亡率较低。[138]适度的运动水平与预防衰老和通过减少炎症潜能改善生活质量有关。[139]运动带来的大部分好处是每周约3500代谢当量(MET)分钟。[140]例如,爬楼梯10分钟,抽真空15分钟,园艺20分钟,跑20分钟,每天步行或骑自行车25分钟,每周可达到约3000 MET分钟。[140]
避免慢性压力(与急性压力相反)与大多数但不是所有研究中的端粒丢失相关,[141] [142]和皮质醇水平降低。长期高皮质醇水平会损害免疫系统,导致心脏损伤/动脉硬化,并与面部衰老有关,而后者反过来又是发病率和死亡率增加的标志。[143] [144]一项荟萃分析显示,孤独感的死亡风险高于吸烟。[145]压力可以通过社会联系,灵性和(男性比女性更清楚)婚姻生活来抵消,所有这些都与长寿有关。[146] [147] [148] [149]
医疗干预
以下药物和干预措施已被证明可以延缓或逆转动物模型中衰老的生物学效应,但尚未证实在人类中这样做。
动物和人类的证据表明,白藜芦醇可能是一种热量限制模拟物。[150]
截至2015年,二甲双胍正在研究其对减缓蠕虫线虫和蟋蟀衰老的潜在影响。[151]它对其他健康人类的影响尚不清楚。[151]
最早,Powers等人在2006年首次证明雷帕霉素可延长真核生物的寿命。他们表现出雷帕霉素对酵母细胞寿命延长的剂量反应作用。[152]在2009年的一项研究中,喂食雷帕霉素的小鼠的寿命从治疗开始时增加了28%至38%,或总体增加9至14%的最大寿命。特别值得注意的是,治疗始于20个月的老鼠,相当于60人年。[153]雷帕霉素随后被证明可以在几个单独的实验中延长小鼠的寿命,[154] [155]并且现在正在非人灵长类动物(mar猴)中为此目的进行测试。[156]
癌症遗传学家Ronald A. DePinho和他的同事发表了对小鼠的研究,其中端粒酶活性首先被遗传去除。然后,在小鼠过早衰老后,它们通过重新激活端粒酶基因来恢复端粒酶活性。结果,小鼠恢复了活力:萎缩的睾丸恢复正常,动物恢复了生育能力。其他器官,如脾脏,肝脏,肠道和大脑,从其退化状态恢复。 “[这一发现]提供了这样一种可能性:通过在已停止工作的细胞中重新唤醒酶,可以减缓正常的人类衰老”,Ronald DePinho说。然而,激活人类端粒酶可能会促进肿瘤的生长。[157]
秀丽隐杆线虫中大多数已知的遗传干预措施使寿命延长1.5至2.5倍。截至2009年,秀丽隐杆线虫的寿命延长记录是单基因突变,使成年人的存活率提高了十倍。[53]模式生物中发现的一些衰老机制的强烈保护意味着它们可能有助于提高人类的生存。但是,好处可能不成比例;秀丽隐杆线虫的寿命增长通常大于果蝇,而果蝇的寿命增长则高于哺乳动物。对此的一种解释是,寿命更长的哺乳动物已经具有许多促进寿命的特性。[53]
研究项目和奖品
一些研究工作旨在减缓衰老并延长健康寿命。[158] [159] [160]
美国国家老龄问题研究所目前资助一项干预测试计划,研究人员提名化合物(基于特定的分子老化理论),以评估它们对远交老鼠的寿命和与年龄相关的生物标志物的影响。[161]哺乳动物以前与年龄相关的测试已经证明在很大程度上是不可再生的,因为动物数量少,饲养条件松弛。[引证需要]干预测试计划旨在通过在三个国际公认的小鼠衰老中心进行平行实验来解决这个问题。 UTHSCSA的Barshop研究所,密歇根大学安娜堡分校和杰克逊实验室。
一些公司和组织,如Google Calico,Human Longevity,Craig Venter,Gero,[162] SENS研究基金会和俄罗斯生命科学扩展,[163]宣布停止或推迟衰老作为他们的目标。
存在延长寿命和减缓哺乳动物衰老的奖品。 Methuselah基金会提供Mprize。最近,推出了价值100万美元的Palo Alto Longevity Prize。这是一项研究激励奖,旨在鼓励来自世界各地的团队全力以赴地“破解规范我们健康和生命的代码”。它由Joon Yun创立。[164] [165] [166] [167] [168]
社会与文化
主要文章:老龄化与社会
An elderly man

一位老人
不同的文化以不同的方式表达年龄。成年人的年龄通常是从出生那天起的整年测量的。设定生命时期的任意分裂可能包括:少年(通过婴儿期,童年,青春期前,青春期),成年早期,成年中期和成年后期。更随意的术语可能包括“青少年”,“青少年”,“二十几岁”,“三十多岁”等,以及“丹麦人”,“维吾尔族”,“三文鱼”,“四人制”等。
大多数法律制度都规定了个人被允许或被迫从事特定活动的特定年龄。这些年龄规格包括投票年龄,饮酒年龄,同意年龄,成年年龄,刑事责任年龄,结婚年龄,候选年龄和强制退休年龄。例如,电影的入场可取决于电影评级系统的年龄。公共汽车票价可能会为年轻人或老年人打折。每个国家,政府和非政府组织都有不同的年龄分类方法。换句话说,按时间顺序排列可以区别于“社会老龄化”(文化年龄 - 人们应该如何随着年龄增长而行动的期望)和“生物老化”(生物体随着年龄增长而变化的身体状态)。[169]
在人口基金关于21世纪老龄化的报告中,它强调需要“发展一种新的以权利为基础的老龄文化,改变对老龄化和老年人的心态和社会态度,从福利接受者到积极的社会贡献成员“[170]人口基金说,”除其他外,“需要努力制定国际人权文书,并将其转化为国家法律和条例,以及对年龄歧视提出质疑并将老年人视为自治主体的肯定措施。”[170] ]老年人的音乐参与有助于维持人际关系,促进成功老龄化。[171]与此同时,老年人可以为社会做出贡献,包括照顾和志愿服务。例如,“一项对移居西班牙的玻利维亚移民的研究发现,69%的移民通常与祖父母一起留在家中。在中国农村,祖父母照顾38%的五岁以下父母上班的孩子在城市。“[170]
经济学
另见:人口老龄化
A map showing median age figures for 2015
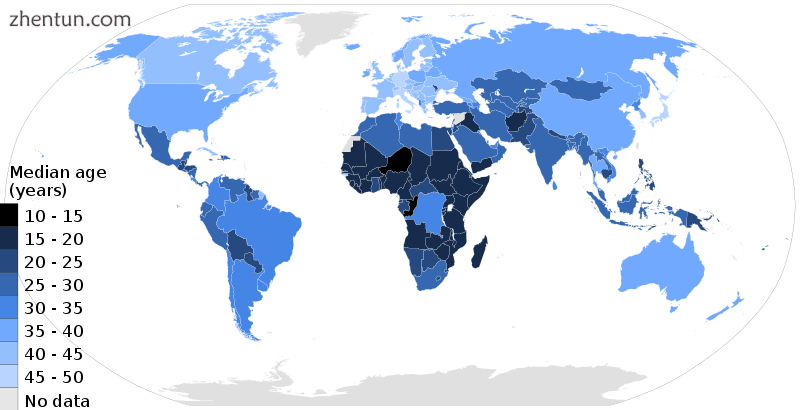
显示2015年年龄中位数的地图
人口老龄化是老年人在社会中的数量和比例的增加。人口老龄化有三个可能的原因:迁移,预期寿命延长(死亡率下降)和出生率下降。老龄化对社会产生重大影响。年轻人往往拥有较少的法律特权(如果他们低于成年年龄),他们更有可能推动政治和社会变革,发展和采用新技术,以及需要教育。老年人对社会和政府有不同的要求,而且往往也有不同的价值观,例如财产和养老金权利。[172]
在21世纪,最重要的人口趋势之一是老龄化。[173]目前,世界上目前人口的11%以上是60岁及以上的人口,联合国人口基金(人口基金)估计,到2050年,这一数字将上升至约22%。[170]由于发展促进了营养,卫生,保健,教育和经济福祉,老龄化已经发生。因此,生育率继续下降,预期寿命有所增加。目前,33个国家的出生时预期寿命超过80岁。老龄化是一种“全球现象”,在发展中国家发生得最快,包括那些拥有大量青年人口的国家,并且可以通过“为个人,家庭和社会提供适当的政策”来克服工作带来的社会和经济挑战。应对这些挑战并从中获益。“[174]
随着发达国家的预期寿命上升和出生率下降,年龄中位数相应上升。据联合国称,这一进程几乎发生在世界上每个国家。[175]年龄中位数上升可能会产生重大的社会和经济影响,因为劳动力逐渐老龄化,老工人和退休人员的数量相对于年轻工人的数量增长。与工作场所的年轻人相比,老年人通常会产生更多与健康相关的费用,而且工人的报酬和养老金负担也会增加。[176]在大多数发达国家,老员工在某种程度上是不可避免的。例如,在美国,劳工统计局估计,到2020年,四分之一的美国工人将达到55岁或以上。[176]
全世界老年人最紧迫的问题是收入保障。这给人口老龄化的政府带来了挑战,以确保继续对养老金体系进行投资,以提供经济独立和减少老年贫困。这些挑战因发展中国家和发达国家而异。人口基金指出,“这些系统的可持续性特别令人关切,特别是在发达国家,而社会保护和养老金覆盖面仍然是发展中国家面临的挑战,发展中国家的大部分劳动力来自非正规部门。 “[170]
全球经济危机增加了财政压力,以确保老年人的经济安全和医疗保健。为了提高这一压力,必须实施社会保护底线,以保障所有老年人的收入保障和获得基本保健和社会服务,并提供有助于推迟残疾和防止老年人贫困的安全网。“[170]
有人认为,人口老龄化已经破坏了经济发展。[177]有证据表明,养老金虽然对老年人的福祉产生影响,但也有利于整个家庭,特别是在危机时期,家庭中可能出现短缺或失业。澳大利亚政府在2003年进行的一项研究估计,“年龄在65岁至74岁之间的妇女每年在无偿护理和志愿工作中每年捐助160亿澳元。同样,同一年龄组的男性每年捐助100亿澳元。”[ 170]
由于老年人口在人口中的比例越来越大,未来几十年医疗保健支出将继续相对于经济增长。这被认为是一种消极现象,应考虑提高劳动生产率等有效策略,以应对老龄化的负面影响。[178]
社会学
在社会学和心理健康领域,老龄化有五种不同的观点:衰老为成熟,衰老为衰退,衰老为生命周期事件,衰老为生成,衰老为生存。[179]与衰老相关的积极因素通常包括经济,就业,婚姻,子女,教育和控制感,以及许多其他因素。衰老的社会科学包括脱离理论,活动理论,选择理论和连续性理论。退休是老年人面临的共同转变,可能会产生积极和消极的后果。[180]由于机器人目前正在崛起,一些理论家认为有必要制定新的老化定义,例如建议对衰老进行生物技术 - 社会定义。[181]
医疗保健需求
随着年龄的增长,不可避免的生物学变化会增加疾病和残疾的风险。人口基金指出,[174]
“医疗保健的生命周期方法 - 早期开始,持续到生育年龄并持续到老年 - 对老年人,甚至所有人的身心健康至关重要。公共政策和计划还应该解决那些无法负担医疗费用的老年贫困人口的需求。“
西欧和日本的许多社会人口老龄化。虽然对社会的影响很复杂,但人们担心对医疗保健需求的影响。文献中提出了大量关于应对老龄化社会长期护理需求预期增长的具体干预措施的建议,可以在四个标题下进行组织:提高系统绩效;重新设计服务;支持非正式照顾者;并改变人口统计参数。[182]
然而,国家卫生支出的年增长主要不是由于人口老龄化需求的增加,而是由于收入增加,昂贵的新医疗技术,卫生保健工作者短缺以及提供者和患者之间的信息不对称所致。 ]随着人们年龄的增长,许多健康问题变得更加普遍。这些包括心理健康问题以及身体健康问题,尤其是痴呆症。
据估计,自1970年以来,人口老龄化仅解释了医疗支出年增长率的0.2个百分点,为4.3%。此外,美国医疗保险制度的某些改革使老年人在家庭保健方面的支出减少了12.5%。年至1996年至2000年。[184]
对衰老的自我认知
对健康的积极自我认知与老年人的幸福感和死亡率降低相关[185] [186]。已经提出了这种关联的各种理由;尽管在控制社会经济地位,心理功能和健康状况的研究中也观察到了这种联系,但客观健康的人自然可以比他们的病人更好地评价自己的健康状况。[187] [186]虽然这种关系并非在所有研究中普遍存在,但在某些情况下可能也是如此。[187]
随着人们年龄的增长,即使客观的健康状况恶化,主观健康仍然相对稳定。[188]事实上,当客观健康受到控制时,感知健康会随着年龄的增长而改善。[189]这种现象被称为“衰老的悖论”。这可能是社会比较的结果; [190]例如,老年人得到的越多,他们认为自己的健康状况就越好于同龄人。[191]老年人经常将其功能和身体衰退与正常衰老过程联系起来。[192] [193]
成功老化
主要文章:成功老化
成功老化的概念可以追溯到20世纪50年代,并在20世纪80年代得到普及。 成功老龄化的传统定义强调缺乏身体和认知障碍。[194] 在他们1987年的文章中,Rowe和Kahn认为成功老龄化涉及三个方面:a)免于疾病和残疾,b)高认知和身体功能,c)社会和生产性参与。[195]
文化参考
古希腊剧作家欧里庇德斯(公元前5世纪)描述了多头的神话怪物Hydra具有再生能力,使其成为长寿的,这是生物属Hydra名称的历史背景。 “约伯记”(约公元前6世纪)描述了人类的生命本质上是有限的,并与砍伐树木在进行植物再生时可能具有的固有永生性进行了比较。[196]
参考:
1. Bowen, Richard L.; Atwood, Craig S. (2004). "Living and Dying for Sex". Gerontology. 50 (5): 265–90. doi:10.1159/000079125. PMID 15331856.
2. Dillin A, Gottschling DE, Nyström T (2014). "The good and the bad of being connected: the integrons of aging". Curr Opin Cell Biol. 26: 107–12. doi:10.1016/j.ceb.2013.12.003. PMC 3927154 Freely accessible. PMID 24529252.
3. Mortimer RK, Johnston JR (1959). "Life Span of Individual Yeast Cells". Nature. 183 (4677): 1751–1752. Bibcode:1959Natur.183.1751M. doi:10.1038/1831751a0. PMID 13666896.
4. Nutman AP, Bennett VC, Friend CR, Van Kranendonk MJ, Chivas AR (2016). "Rapid emergence of life shown by discovery of 3,700-million-year-old microbial structures". Nature. 537 (7621): 535–538. Bibcode:2016Natur.537..535N. doi:10.1038/nature19355. PMID 27580034.
5. Rose MR (1991). Evolutionary Biology of Aging. New York: Oxford University Press.
6. Partridge L, Barton NH (1993). "Optimality, mutation and the evolution of ageing". Nature. 362 (6418): 305–311. Bibcode:1993Natur.362..305P. doi:10.1038/362305a0. PMID 8455716.
7. Williams, George C. (1957). " leiotropy, Natural Selection, and the Evolution of Senescence". Evolution. 11 (4): 398–411. doi:10.2307/2406060. JSTOR 2406060. Lay summary. leiotropy, Natural Selection, and the Evolution of Senescence". Evolution. 11 (4): 398–411. doi:10.2307/2406060. JSTOR 2406060. Lay summary.
8. Stewart EJ, Madden R, Paul G, Taddei F (2005). "Aging and death in an organism that reproduces by morphologically symmetric division". PLoS Biology. 3 (2): e45. doi:10.1371/journal.pbio.0030045. PMC 546039 Freely accessible. PMID 15685293.
9. Pereira-Smith OM, Ning Y (1992). "Molecular genetic studies of cellular senescence". Exp Gerontol. 27 (5–6): 519–22. PMID 1426085.
10. Forster P, Hohoff C, Dunkelmann B, Schürenkamp M, Pfeiffer H, Neuhuber F, Brinkmann B (2015). "Elevated germline mutation rate in teenage fathers". Proc R Soc B. 282 (1803): 1–6. doi:10.1098/rspb.2014.2898. PMC 4345458 Freely accessible. PMID 25694621.
11. Wakayama S, Kohda T, Obokata H, Tokoro M, Li C, Terashita Y, Mizutani E, Nguyen VT, Kishigami S, Ishino F, Wakayama T (2013). "Successful serial recloning in the mouse over multiple generations". Cell Stem Cell. 12 (3): 293–297. doi:10.1016/j.stem.2013.01.005. PMID 23472871.
12. "Does Human Life Span Really Have a Limit?". WebMD. 28 June 2018.
13. Stephen Moss (July 2013). "Big ears: they really do grow as we age". MeshID 000375; OMIM:502000. Retrieved 9 September 2016. 000375; OMIM:502000. Retrieved 9 September 2016.
14. Gerasimov, I.G.; Ignatov, D.Yu. (2004). "Age Dynamics of Body Mass and Human Lifespan". Journal of Evolutionary Biochemistry and Physiology. 40 (3): 343–349. doi:10.1023/B:JOEY.0000042639.72529.e1.
15. Rodriguez Valiente A, Trinidad A, Garcia Berrocal JR, Gorriz C, Ramirez Camacho R (April 2014). "Review: Extended high-frequency (9–20 kHz) audiometry reference thresholds in healthy subjects". Int J Audiol. 53 (8): 531–545. doi:10.3109/14992027.2014.893375. PMID 24749665.
16. Desjardins, Richard; Warnke, Arne Jonas (2012). "Ageing and Skills". OECD Education Working Papers. doi:10.1787/5k9csvw87ckh-en.
17. Finkel, Deborah; Reynolds, Chandra A. (9 July 2013). "Behavior Genetics of Cognition Across the Lifespan". Springer Science & Business Media – via Google Books.
18. Thurstan SA, Gibbs NK, Langton AK, Griffiths CE, Watson RE, Sherratt MJ (2012). "Chemical consequences of cutaneous photoageing". Chem Cent J. 6 (1): 34. doi:10.1186/1752-153X-6-34. PMC 3410765 Freely accessible. PMID 22534143.
19. pmhdev (25 March 2015). "Infertility: Overview" – via www.ncbi.nlm.nih.gov.
20. "Facts About Presbyopia". Last Reviewed October 2010: National Eye Institute. Retrieved 11 September 2016.
21. Weale RA (2003). "Epidemiology of refractive errors and presbyopia". Surv Ophthalmol. 48 (5): 515–43. doi:10.1016/S0039-6257(03)00086-9. PMID 14499819.
22. Truscott RJ (2009). " resbyopia. Emerging from a blur towards an understanding of the molecular basis for this most common eye condition". Exp Eye Res. 88 (2): 241–7. doi:10.1016/j.exer.2008.07.003. PMID 18675268. resbyopia. Emerging from a blur towards an understanding of the molecular basis for this most common eye condition". Exp Eye Res. 88 (2): 241–7. doi:10.1016/j.exer.2008.07.003. PMID 18675268.
23. Pathai, S; Shiels, PG; Lawn, SD; Cook, C; Gilbert, C (March 2013). "The eye as a model of ageing in translational research--molecular, epigenetic and clinical aspects". Ageing research reviews. 12 (2): 490–508. doi:10.1016/j.arr.2012.11.002. PMID 23274270.
24. Pandhi, D; Khanna, D (2013). " remature graying of hair". Indian journal of dermatology, venereology and leprology. 79 (5): 641–53. doi:10.4103/0378-6323.116733. PMID 23974581. remature graying of hair". Indian journal of dermatology, venereology and leprology. 79 (5): 641–53. doi:10.4103/0378-6323.116733. PMID 23974581.
25. Hamilton, J. B. (1 March 1951). " atterned loss of hair in man; types and incidence". Annals of the New York Academy of Sciences. 53 (3): 708–728. Bibcode:1951NYASA..53..708H. doi:10.1111/j.1749-6632.1951.tb31971.x. PMID 14819896. atterned loss of hair in man; types and incidence". Annals of the New York Academy of Sciences. 53 (3): 708–728. Bibcode:1951NYASA..53..708H. doi:10.1111/j.1749-6632.1951.tb31971.x. PMID 14819896.
26. Vary JC, Jr (November 2015). "Selected Disorders of Skin Appendages--Acne, Alopecia, Hyperhidrosis". The Medical clinics of North America. 99 (6): 1195–211. doi:10.1016/j.mcna.2015.07.003. PMID 26476248.
27. Takahashi, TA; Johnson, KM (May 2015). "Menopause". The Medical clinics of North America. 99 (3): 521–34. doi:10.1016/j.mcna.2015.01.006. PMID 25841598.
28. Thomas, Elaine; Peat, George; Croft, Peter (2014). "Defining and mapping the person with osteoarthritis for population studies and public health". Rheumatology (Oxford). 53 (2): 338–345. doi:10.1093/rheumatology/ket346. PMC 3894672 Freely accessible. PMID 24173433.
29. "Hearing Loss and Older Adults" (Last Updated June 3, 2016). National Institute on Deafness and Other Communication Disorders. Retrieved September 11, 2016.
30 Rubel, Edwin W.; Furrer, Stephanie A.; Stone, Jennifer S. (2013). "Review: A brief history of hair cell regeneration research and speculations on the future". Hearing Research. 297: 42–51. doi:10.1016/j.heares.2012.12.014. PMC 3657556 Freely accessible. PMID 23321648.
31. "Facts About Cataract". September 2015. Retrieved 14 August 2016.
32. Fried, LP; Tangen, CM; Walston, J; Newman, AB; Hirsch, C; Gottdiener, J; Seeman, T; Tracy, R; Kop, WJ; Burke, G; McBurnie, MA (Mar 2001). "Frailty in older adults: evidence for a phenotype". The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 56 (3): M146–56. doi:10.1093/gerona/56.3.m146. PMID 11253156.
33. Percentage derived from Table 2 in Fried et al. 2001
34. Wang JC, Bennett M (2012). "Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence". Circ Res. 111 (2): 245–59. doi:10.1161/CIRCRESAHA.111.261388. PMID 22773427.
35. Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S (2016). "Epidemiology of Atherosclerosis and the Potential to Reduce the Global Burden of Atherothrombotic Disease". Circ Res. 118 (4): 535–46. doi:10.1161/CIRCRESAHA.115.307611. PMID 26892956.
36. {https://www.heart.org/idc/groups ... able/ucm_470704.pdf}
37. Zimmer, Carl (October 5, 2016). "What's the Longest Humans Can Live? 115 Years, New Study Says". The New York Times. Retrieved October 6, 2016.
38. Dong, Xiao; Milholland, Brandon; Vijg, Jan (October 5, 2016). "Evidence for a limit to human lifespan". Nature. 538 (7624): 257–259. Bibcode:2016Natur.538..257D. doi:10.1038/nature19793. PMID 27706136. Retrieved October 6, 2016.
39. Larson, EB; Yaffe, K; Langa, KM (12 December 2013). "New insights into the dementia epidemic". The New England Journal of Medicine. 369 (24): 2275–7. doi:10.1056/nejmp1311405. PMC 4130738 Freely accessible. PMID 24283198.
40. Umphred, Darcy (2012). Neurological rehabilitation (6th ed.). St. Louis, Mo.: Elsevier Mosby. p. 838. ISBN 978-0-323-07586-2.
41. Schaie, K. Warner (2005). Developmental Influences on Adult Intelligence. doi:10.1093/acprof so/9780195156737.001.0001. ISBN 978-0-19-515673-7.[page needed] so/9780195156737.001.0001. ISBN 978-0-19-515673-7.[page needed]
42. Stuart-Hamilton, Ian (2006). The Psychology of Ageing: An Introduction. London: Jessica Kingsley Publishers. ISBN 1-84310-426-1.
43. Marner, Lisbeth; Nyengaard, Jens R.; Tang, Yong; Pakkenberg, Bente (2003). "Marked loss of myelinated nerve fibers in the human brain with age". The Journal of Comparative Neurology. 462 (2): 144–52. doi:10.1002/cne.10714. PMID 12794739.
44. Peters, Alan (1 January 2007). Riddle, David R., ed. Brain Aging: Models, Methods, and Mechanisms. CRC Press/Taylor & Francis. PMID 21204349.
45. Worrall, L.,& Hickson, L. M. (2003). "Theoretical foundations of communication disability in aging", pp. 32–33 in Linda E. Worrall & Louise M. Hickson(Eds.). Communication disability in aging: from prevention to intervention. Clifton Park, NY: Delmar Learning
46. Mehta, S (September 2015). "Age-Related Macular Degeneration". Primary care. 42 (3): 377–91. doi:10.1016/j.pop.2015.05.009. PMID 26319344.
47. Nussbaum, J. F., Thompson, T. L., & Robinson, J. D. (1989). "Barriers to conversation", pp. 234–253 in Jon F. Nussbaum, Teresa Thompson, James D. Robinson (Eds.). Communication and aging. New York: Harper & Row
48. De Grey, Aubrey D.N.J (2007). "Life Span Extension Research and Public Debate: Societal Considerations". Studies in Ethics, Law, and Technology. 1. doi:10.2202/1941-6008.1011.
49. Lopez, Alan D; Mathers, Colin D; Ezzati, Majid; Jamison, Dean T; Murray, Christopher JL (2006). "Global and regional burden of disease and risk factors, 2001: Systematic analysis of population health data". The Lancet. 367 (9524): 1747–57. doi:10.1016/S0140-6736(06)68770-9. PMID 16731270.
50. Brunet Lab: Molecular Mechanisms of Longevity and Age Related Diseases. Stanford.edu. Retrieved on 11 April 2012.
51. Janssens GE, Meinema AC, González J, Wolters JC, Schmidt A, Guryev V, Bischoff R, Wit EC, Veenhoff LM, Heinemann M (2015). " rotein biogenesis machinery is a driver of replicative aging in yeast". ELife. 4: e08527. doi:10.7554/eLife.08527. PMC 4718733 Freely accessible. PMID 26422514. rotein biogenesis machinery is a driver of replicative aging in yeast". ELife. 4: e08527. doi:10.7554/eLife.08527. PMC 4718733 Freely accessible. PMID 26422514.
52. Deepti S. Wilkinson; Rebecca C. Taylor; Andrew Dillin (2012). "Analysis of Aging in Caenorhabditis elegans". In Joel H. Rothman; Andrew Singson. Caenorhabditis Elegans: Cell Biology and Physiology. Academic Press. pp. 353–381. ISBN 978-0-12-394620-1.
53. Shmookler Reis RJ, Bharill P, Tazearslan C, Ayyadevara S (2009). "Extreme-longevity mutations orchestrate silencing of multiple signaling pathways". Biochim Biophys Acta. 1790 (10): 1075–83. doi:10.1016/j.bbagen.2009.05.011. PMC 2885961 Freely accessible. PMID 19465083.
54. "Mitochondrial Theory of Aging and Other Aging Theories". 1Vigor. Retrieved 4 October 2013.
55. Jin, Kunlin (2010). "Modern Biological Theories of Aging". Aging Dis. 1 (2): 72–74. PMC 2995895 Freely accessible. PMID 21132086.
56. Lopez-Otin, C; Blasco, MA; Partridge, L; Serrano, M; Kroemer, G (2013). "The hallmarks of aging". Cell. 153 (6): 1194–1217. doi:10.1016/j.cell.2013.05.039. PMC 3836174 Freely accessible. PMID 23746838.
57. Guarente, Leonard P.; Partridge, Linda; Wallace, Douglas C. (2008). Molecular Biology of Aging. New York: Cold Spring Harbor. pp. 347–362. ISBN 978-0-87969-824-9.
58. Taylor RC, Dillin A (2011). "Aging as an event of proteostasis collapse". Cold Spring Harb Perspect Biol. 3 (5): a004440. doi:10.1101/cshperspect.a004440. PMC 3101847 Freely accessible. PMID 21441594.
59. Melzer, S; Lens, F; Gennen, J; Vanneste, S; Rohde, A; Beeckman, T (2008). "Flowering-time genes modulate meristem determinacy and growth form in Arabidopsis thaliana". Nature Genetics. 40 (12): 1489–92. doi:10.1038/ng.253. PMID 18997783.
60. Chesterton, Marnie (12 June 2017). "The oldest living thing on Earth". bbc.com. Retrieved 16 September 2017.
61. "Oldlist". Rocky Mountain Tree Ring Research. Retrieved 2016-08-12.
62. Danuta Sosnowska, Chris Richardson, William E. Sonntag, Anna Csiszar, Zoltan Ungvari, Iain Ridgway (2014). "A Heart That Beats for 500 Years: Age-Related Changes in Cardiac Proteasome Activity, Oxidative Protein Damage and Expression of Heat Shock Proteins, Inflammatory Factors, and Mitochondrial Complexes in Arctica islandica, the Longest-Living Noncolonial Animal". J Gerontol a Biol Sci Med Sci. 69 (12): 1448–61. doi:10.1093/gerona/glt201. PMC 4271020 Freely accessible. PMID 24347613.
63. Julius Nielsen, Rasmus B. Hedeholm, Jan Heinemeier, Peter G. Bushnell, Jørgen S. Christiansen, Jesper Olsen, Christopher Bronk Ramsey, Richard W. Brill, Malene Simon, Kirstine F. Steffensen, John F. Steffensen (2016). "Eye lens radiocarbon reveals centuries of longevity in the Greenland shark (Somniosus microcephalus)". Science. 353 (6300): 702–704. Bibcode:2016Sci...353..702N. doi:10.1126/science.aaf1703. PMID 27516602.
64. Durkin, A., Fisher, C.R. & Cordes, E.E. (2017). "Extreme longevity in a deep-sea vestimentiferan tubeworm and its implications for the evolution of life history strategies". The Science of Nature. 104: 63. Bibcode:2017SciNa.104...63D. doi:10.1007/s00114-017-1479-z. PMID 28689349.
65. Timiras, Paola S. (2003) Physiological Basis of Ageing and Geriatrics. Informa Health Care. ISBN 0-8493-0948-4. p. 26.
66. Silverman, Jacob. "Is there a 400 pound lobster out there?". howstuffworks.
67. Wallace, David Foster (2005). Consider the Lobster and Other Essays. Little, Brown & Company. ISBN 0-316-15611-6.[page needed]
68. Guerin, J (2004). "Emerging area of aging research: long-lived animals with "negligible senescence"". Ann N Y Acad Sci. 1019: 518–20. Bibcode:2004NYASA1019..518G. doi:10.1196/annals.1297.096. PMID 15247078.
69. Bartke A (2011). "Single-gene mutations and healthy ageing in mammals". Philos Trans R Soc Lond B Biol Sci. 366 (1561): 28–34. doi:10.1098/rstb.2010.0281. PMC 3001310 Freely accessible. PMID 21115527.
70. Berdyshev, G; Korotaev, G; Boiarskikh, G; Vaniushin, B (1967). "Nucleotide composition of DNA and RNA from somatic tissues of humpback and its changes during spawning". Biokhimiia. 31: 88–993.
71. Marioni, R; Shah, S; McRae, A; Chen, B; Colicino, E; Harris, S; Gibson, J; Henders, A; Redmond, P; Cox, S; Pattie, A; Corley, J; Murphy, L; Martin, N; Montgomery, G; Feinberg, A; Fallin, M; Multhaup, M; Jaffe, A; Joehanes, R; Schwartz, J; Just, A; Lunetta, K; Murabito, JM; Starr, J; Horvath, S; Baccarelli, A; Levy, D; Visscher, P; Wray, N; Deary, I (2015). "DNA methylation age of blood predicts all-cause mortality in later life". Genome Biology. 16 (1): 25. doi:10.1186/s13059-015-0584-6. PMC 4350614 Freely accessible. PMID 25633388.
72. Christiansen, L (2015). "DNA methylation age is associated with mortality in a longitudinal Danish twin study". Aging Cell. 15 (1): 149–154. doi:10.1111/acel.12421. PMC 4717264 Freely accessible. PMID 26594032.
73. Horvath, S (2015). "Decreased epigenetic age of PBMCs from Italian semi-supercentenarians and their offspring". Aging (Dec).
74. Ocampo, A.; et al. (2016). "In Vivo Amelioration of Age-Associated Hallmarks by Partial Reprogramming". Cell. 167 (7): 1719–1733. doi:10.1016/j.cell.2016.11.052. PMID 27984723.
75. Tina Hesman Saey (15 December 2016). " roteins that reprogram cells can turn back mice's aging clock". Retrieved 19 December 2016. roteins that reprogram cells can turn back mice's aging clock". Retrieved 19 December 2016.
76. Stibich, Mark (19 April 2009) Telomere Shortening – The Secret to Aging?. About.com
77. m. Mikhelson, Victor; Gamaley, Irina (2013). "Telomere Shortening is a Sole Mechanism of Aging in Mammals". Current Aging Science. 5 (3): 203–8. doi:10.2174/1874609811205030006. PMID 23387887.
78. Hayflick, L. (1987) Origins of longevity. In Warner, H.R., Butler, R.N., Sprott, R.L. and Schneider, E.L. (eds), Modern Biological Theories of Aging. Raven Press, New York, pp. 21–34. ISBN 0-88167-310-2
79. Bernstein C, Bernstein H. (1991) Aging, Sex, and DNA Repair. Academic Press, San Diego. ISBN 0-12-092860-4. pp. 314, 320 and 326
80. Hemann, M. T.; Greider, CW (2000). "Wild-derived inbred mouse strains have short telomeres". Nucleic Acids Research. 28 (22): 4474–8. doi:10.1093/nar/28.22.4474. PMC 113886 Freely accessible. PMID 11071935.
81. Blasco, María A; Lee, Han-Woong; Hande, M.Prakash; Samper, Enrique; Lansdorp, Peter M; Depinho, Ronald A; Greider, Carol W (1997). "Telomere Shortening and Tumor Formation by Mouse Cells Lacking Telomerase RNA". Cell. 91 (1): 25–34. doi:10.1016/S0092-8674(01)80006-4. PMID 9335332.
82. Kipling, David; Cooke, Howard J. (1990). "Hypervariable ultra-long telomeres in mice". Nature. 347 (6291): 400–2. Bibcode:1990Natur.347..400K. doi:10.1038/347400a0. PMID 2170845.
83. Nordfjäll, K; Svenson, U; Norrback, K. F.; Adolfsson, R; Lenner, P; Roos, G (2009). "The Individual Blood Cell Telomere Attrition Rate is Telomere Length Dependent". PLoS Genetics. 5 (2): e1000375. doi:10.1371/journal.pgen.1000375. PMC 2633043 Freely accessible. PMID 19214207.
84. Willcox B. J. (2008). "FOXO3A genotype is strongly associated with human longevity". PNAS. 105 (37): 13987–13992. Bibcode:2008PNAS..10513987W. doi:10.1073/pnas.0801030105. PMC 2544566 Freely accessible. PMID 18765803.
85. Flachsbart F, Caliebe A, Kleindorp R, Blanché H, von Eller-Eberstein H, Nikolaus S, Schreiber S, Nebel A (2009). "Association of FOXO3A variation with human longevity confirmed in German centenarians". PNAS. 106 (8): 2700–5. Bibcode:2009PNAS..106.2700F. doi:10.1073/pnas.0809594106. PMC 2650329 Freely accessible. PMID 19196970.
86. Ghosh, H. S.; McBurney, M; Robbins, P. D. (2010). "SIRT1 Negatively Regulates the Mammalian Target of Rapamycin". PLoS ONE. 5 (2): e9199. Bibcode:2010PLoSO...5.9199G. doi:10.1371/journal.pone.0009199. PMC 2821410 Freely accessible. PMID 20169165.
84. Fontana L, Partridge L, Longo VD (2010). "Extending healthy life span—from yeast to humans". Science. 328 (5976): 321–6. Bibcode:2010Sci...328..321F. doi:10.1126/science.1172539. PMC 3607354 Freely accessible. PMID 20395504.
88. Johnson, Simon C.; Rabinovitch, Peter S.; Kaeberlein, Matt (2013). "MTOR is a key modulator of ageing and age-related disease". Nature. 493 (7432): 338–45. Bibcode:2013Natur.493..338J. doi:10.1038/nature11861. PMC 3687363 Freely accessible. PMID 23325216.
89. Halicka HD1, Zhao H, Li J, Lee YS, Hsieh TC, Wu JM, Darzynkiewicz Z. (2012) Potential anti-aging agents suppress the level of constitutive mTOR- and DNA damage- signaling. Aging (Albany NY). 2012 Dec;4(12):952-65. PMID 23363784, PMC 3615161
90. Junnila, RK; List, EO; Berryman, DE; Murrey, JW; Kopchick, JJ (2013). "The GH/IGF-1 axis in ageing and longevity". Nat Rev Endocrinol. 9 (6): 366–76. doi:10.1038/nrendo.2013.67. PMC 4074016 Freely accessible. PMID 23591370.
91. J Sun; Kale, SP; Childress, AM; Pinswasdi, C; Jazwinski, SM (15 July 1994). "Divergent roles of RAS1 and RAS2 in yeast longevity". Journal of Biological Chemistry. 269 (28): 18638–45. PMID 8034612.
92. Wei M, Fabrizio P, Hu J, Ge H, Cheng C, Li L, Longo VD (2008). "Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9". PLoS Genet. 4 (1): 139–149. doi:10.1371/journal.pgen.0040013. PMC 2213705 Freely accessible. PMID 18225956.
93. "10-Fold Life Span Extension Reported". University of Southern California.
94. Nyström, T. (2003). "The free-radical hypothesis of aging goes prokaryotic". Cellular and Molecular Life Sciences (CMLS). 60 (7): 1333–41. doi:10.1007/s00018-003-2310-X. PMID 12943222.
95. Kang, H.-J.; Feng, Z.; Sun, Y.; Atwal, G.; Murphy, M. E.; Rebbeck, T. R.; Rosenwaks, Z.; Levine, A. J.; Hu, W. (2009). "Single-nucleotide polymorphisms in the p53 pathway regulate fertility in humans". Proceedings of the National Academy of Sciences. 106 (24): 9761–6. Bibcode:2009PNAS..106.9761K. doi:10.1073/pnas.0904280106. PMC 2700980 Freely accessible. PMID 19470478.
96. Smith, K. R.; Hanson, H. A.; Mineau, G. P.; Buys, S. S. (2011). "Effects of BRCA1 and BRCA2 mutations on female fertility". Proceedings of the Royal Society B: Biological Sciences. 279 (1732): 1389–95. doi:10.1098/rspb.2011.1697. PMC 3282366 Freely accessible. PMID 21993507.
97. Atwood, Craig S.; Bowen, Richard L. (2011). "The reproductive-cell cycle theory of aging: An update". Experimental Gerontology. 46 (2–3): 100–7. doi:10.1016/j.exger.2010.09.007. PMID 20851172.
98. Mair W, Steffen KK, Dillin A (2011). "SIP-ing the elixir of youth". Cell. 146 (6): 859–60. doi:10.1016/j.cell.2011.08.026. PMID 21925309.
99. Gensler, Helen L.; Bernstein, Harris (1981). "DNA Damage as the Primary Cause of Aging". The Quarterly Review of Biology. 56 (3): 279–303. doi:10.1086/412317. JSTOR 2826464. PMID 7031747.
100. Sinha, Jitendra Kumar; Ghosh, Shampa; Swain, Umakanta; Giridharan, Nappan Veethil; Raghunath, Manchala (2014). "Increased macromolecular damage due to oxidative stress in the neocortex and hippocampus of WNIN/Ob, a novel rat model of premature aging". Neuroscience. 269: 256–64. doi:10.1016/j.neuroscience.2014.03.040. PMID 24709042.
101. Freitas, Alex A.; De Magalhães, João Pedro (2011). "A review and appraisal of the DNA damage theory of ageing". Mutation Research/Reviews in Mutation Research. 728 (1–2): 12–22. doi:10.1016/j.mrrev.2011.05.001. PMID 21600302.
102. L. Robert; J. Labat-Robert; A. M. Robert (2010). "Genetic, epigenetic and posttranslational mechanisms of aging". Biogerontology. 11 (4): 387–399. doi:10.1007/s10522-010-9262-y. PMID 20157779.
103. Strehler, Bernard L. (1986). "Genetic instability as the primary cause of human aging". Experimental Gerontology. 21 (4–5): 283–319. doi:10.1016/0531-5565(86)90038-0. PMID 3545872.
104. Gavrilov, L. A.; Gavrilova, N. S. (2006), "Reliability Theory of Aging and Longevity", pp. 3–42 in Handbook of the Biology of Aging, ed. Masoro E. J. and Austad S. N, Academic Press, San Diego, CA.
105. Carroll B, Hewitt G, Korolchuk VI (2013). "Autophagy and ageing: implications for age-related neurodegenerative diseases". Essays Biochem. 55: 119–31. doi:10.1042/bse0550119. PMID 24070476.
106. Cui M, Yu H, Wang J, Gao J, Li J (2013). "Chronic Caloric Restriction and Exercise Improve Metabolic Conditions of Dietary-Induced Obese Mice in Autophagy Correlated Manner without Involving AMPK". J Diabetes Res. 2013: 852754. doi:10.1155/2013/852754. PMC 3671310 Freely accessible. PMID 23762877.
107. Jin K (2010). "Modern Biological Theories of Aging". Aging Dis. 1 (2): 72–74. PMC 2995895 Freely accessible. PMID 21132086.
108. Bjorksten, Johan; Tenhu, Heikki (1990). "The crosslinking theory of aging — Added evidence". Experimental Gerontology. 25 (2): 91–5. doi:10.1016/0531-5565(90)90039-5. PMID 2115005.
109. Trifunovic, A.; Larsson, N.-G. (2008-02-01). "Mitochondrial dysfunction as a cause of ageing". Journal of Internal Medicine. 263 (2): 167–178. doi:10.1111/j.1365-2796.2007.01905.x. ISSN 1365-2796.
110. Harman, D. (1981). "The aging process". Proceedings of the National Academy of Sciences. 78 (11): 7124–8. Bibcode:1981PNAS...78.7124H. doi:10.1073/pnas.78.11.7124. PMC 349208 Freely accessible. PMID 6947277.
111. Schulz, Tim J.; Zarse, Kim; Voigt, Anja; Urban, Nadine; Birringer, Marc; Ristow, Michael (2007). "Glucose Restriction Extends Caenorhabditis elegans Life Span by Inducing Mitochondrial Respiration and Increasing Oxidative Stress". Cell Metabolism. 6 (4): 280–93. doi:10.1016/j.cmet.2007.08.011. PMID 17908557.
112. Hamilton ML, Van Remmen H, Drake JA, Yang H, Guo ZM, Kewitt K, Walter CA, Richardson A (2001). "Does oxidative damage to DNA increase with age?". Proc. Natl. Acad. Sci. U.S.A. 98 (18): 10469–74. Bibcode:2001PNAS...9810469H. doi:10.1073/pnas.171202698. PMC 56984 Freely accessible. PMID 11517304.
113. Wolf FI, Fasanella S, Tedesco B, Cavallini G, Donati A, Bergamini E, Cittadini A (2005). " eripheral lymphocyte 8-OHdG levels correlate with age-associated increase of tissue oxidative DNA damage in Sprague-Dawley rats. Protective effects of caloric restriction". Exp. Gerontol. 40 (3): 181–8. doi:10.1016/j.exger.2004.11.002. PMID 15763395. eripheral lymphocyte 8-OHdG levels correlate with age-associated increase of tissue oxidative DNA damage in Sprague-Dawley rats. Protective effects of caloric restriction". Exp. Gerontol. 40 (3): 181–8. doi:10.1016/j.exger.2004.11.002. PMID 15763395.
114. Anson, R. M.; Bohr, V. A. (October 2000). "Mitochondria, oxidative DNA damage, and aging". Journal of the American Aging Association. 23 (4): 199–218. doi:10.1007/s11357-000-0020-y. ISSN 2152-4041. PMC 3455271 Freely accessible. PMID 23604866.
115. Guarente L, Picard F (2005). "Calorie restriction—the SIR2 connection". Cell. 120 (4): 473–82. doi:10.1016/j.cell.2005.01.029. PMID 15734680.
116. Agarwal B, Baur JA (2011). "Resveratrol and life extension". Ann N Y Acad Sci. 1215 (1): 138–43. Bibcode:2011NYASA1215..138A. doi:10.1111/j.1749-6632.2010.05850.x. PMID 21261652.
117. Larson-Meyer DE; et al. (2008). "Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function". Obesity (Silver Spring). 16 (6): 1355–1362. doi:10.1038/oby.2008.201. PMC 2748341 Freely accessible.
118. Heilbronn LK; et al. (2006). "Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial". JAMA. 295: 1539–1548. doi:10.1001/jama.295.13.1539. PMC 2692623 Freely accessible.
119. Colman RJ, Anderson RM, Johnson SC, Kastman EK, et al. (2009). "Caloric restriction delays disease onset and mortality in rhesus monkeys". Science. 325 (5937): 201–4. Bibcode:2009Sci...325..201C. doi:10.1126/science.1173635. PMC 2812811 Freely accessible. PMID 19590001.
120. Mattison, Julie A.; et al. (2012). "Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study". Nature. 489 (7415): 318–321. Bibcode:2012Natur.489..318M. doi:10.1038/nature11432. PMC 3832985 Freely accessible. PMID 22932268.
121. Colman RJ; Beasley TM; Kemnitz JW; Johnson SC; Weindruch R; Anderson RM (Apr 1, 2014). "Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys". Nature Communications. 5: 3557. Bibcode:2014NatCo...5E3557C. doi:10.1038/ncomms4557. PMC 3988801 Freely accessible. PMID 24691430. Retrieved 30 April 2014.
122. "There may be little advantage of moderate CR over modest CR—this would be an extremely important discovery and one that merits further investigation."
123. Hayflick, Leonard. (1994). How and why we age. New York: Ballantine Books. p. 261. ISBN 978-0-345-33918-8. OCLC 29908633.
124. Bowman K; Delgado J; Henley WE; Masoli JA; Kos K; Brayne C; Thokala P; Lafortune L; Kuchel GA; Ble A; Melzer D; Ageing Well Programme of the NIHR School for Public Health Research, England (Aug 4, 2016). "Obesity in Older People With and Without Conditions Associated With Weight Loss: Follow-up of 955,000 Primary Care Patients". J Gerontol a Biol Sci Med Sci. 72 (2): 203–209. doi:10.1093/gerona/glw147. PMC 5233914 Freely accessible. PMID 27492450.
125. Nakagawa, Shinichi; Lagisz, Malgorzata; Hector, Katie L.; Spencer, Hamish G. (2012-06-01). "Comparative and meta-analytic insights into life extension via dietary restriction". Aging Cell. 11 (3): 401–409. doi:10.1111/j.1474-9726.2012.00798.x. ISSN 1474-9726. PMID 22268691.
126. Simpson, Stephen J.; Raubenheimer, David (2009-10-22). "Macronutrient balance and lifespan". Aging. 1 (10): 875–880. doi:10.18632/aging.100098. ISSN 1945-4589. PMC 2815731 Freely accessible. PMID 20157561.
127. Melnik, Bodo C. (2012-03-15). "Leucine signaling in the pathogenesis of type 2 diabetes and obesity". World Journal of Diabetes. 3 (3): 38–53. doi:10.4239/wjd.v3.i3.38. ISSN 1948-9358. PMC 3310004 Freely accessible. PMID 22442749.
128. Yan, Lijun; Lamb, Richard F. (2012-08-01). "Amino acid sensing and regulation of mTORC1". Seminars in Cell & Developmental Biology. 23 (6): 621–625. doi:10.1016/j.semcdb.2012.02.001. ISSN 1096-3634. PMID 22342805.
129. Rees, K; Hartley, L; Flowers, N; Clarke, A; Hooper, L; Thorogood, M; Stranges, S (12 August 2013). "'Mediterranean' dietary pattern for the primary prevention of cardiovascular disease". The Cochrane Database of Systematic Reviews. 8 (8): CD009825. doi:10.1002/14651858.CD009825.pub2. PMC 4176656 Freely accessible. PMID 23939686.
130. Sofi F, Cesari F, Abbate R, Gensini GF, Casini A (2008). "Adherence to Mediterranean diet and health status: meta-analysis". BMJ (Clinical research ed.). 337 (sep11 2): a1344. doi:10.1136/bmj.a1344. PMC 2533524 Freely accessible. PMID 18786971.
131. de Gaetano, Giovanni (2016-08-29). "Mediterranean diet associated with lower risk of early death in cardiovascular disease patients. European Society of Cardiology". ScienceDaily.
132. Rhonda Rowland (15 February 2002). "Experts challenge study linking sleep, life span". CNN. Retrieved 29 October 2013.
133. Patel SR, Ayas NT, Malhotra MR, White DP, Schernhammer ES, Speizer FE, Stampfer MJ, Hu FB (May 2004). "A prospective study of sleep duration and mortality risk in women". Sleep. 27 (3): 440–4. doi:10.1093/sleep/27.3.440. PMID 15164896.
134. Ferrie JE, Shipley MJ, Cappuccio FP, Brunner E, Miller MA, Kumari M, Marmot MG (December 2007). "A prospective study of change in sleep duration: associations with mortality in the Whitehall II cohort". Sleep. 30 (12): 1659–66. doi:10.1093/sleep/30.12.1659. PMC 2276139 Freely accessible. PMID 18246975. Lay summary – University of Warwick.
135. Patel SR, Malhotra A, Gottlieb DJ, White DP, Hu FB (July 2006). "Correlates of long sleep duration". Sleep. 29 (7): 881–9. doi:10.1093/sleep/29.7.881. PMC 3500381 Freely accessible. PMID 16895254.; cf. Irwin MR, Ziegler M (February 2005). "Sleep deprivation potentiates activation of cardiovascular and catecholamine responses in abstinent alcoholics". Hypertension. 45 (2): 252–7. doi:10.1161/01.HYP.0000153517.44295.07. PMID 15642774.
136. Yetish G, Kaplan H, Gurven M, Wood B, Pontzer H, Manger PR, Wilson C, McGregor R, Siegel JM (November 2015). "Natural sleep and its seasonal variations in three pre-industrial societies". Curr. Biol. 25 (21): 2862–8. doi:10.1016/j.cub.2015.09.046. PMC 4720388 Freely accessible. PMID 26480842.
137. Gremeaux, V; Gayda, M; Lepers, R; Sosner, P; Juneau, M; Nigam, A (December 2012). "Exercise and longevity". Maturitas. 73 (4): 312–7. doi:10.1016/j.maturitas.2012.09.012. PMID 23063021.
138. Department Of Health And Human Services, United States (1996). " hysical Activity and Health". United States Department of Health. ISBN 9781428927940. hysical Activity and Health". United States Department of Health. ISBN 9781428927940.
139. Woods, Jeffrey A.; Wilund, Kenneth R.; Martin, Stephen A.; Kistler, Brandon M. (2011-10-29). "Exercise, Inflammation and Aging". Aging and Disease. 3 (1): 130–140. ISSN 2152-5250. PMC 3320801 Freely accessible. PMID 22500274.
140. Kyu, Hmwe H; Bachman, Victoria F; Alexander, Lily T; Mumford, John Everett; Afshin, Ashkan; Estep, Kara; Veerman, J Lennert; Delwiche, Kristen; Iannarone, Marissa L; Moyer, Madeline L; Cercy, Kelly; Vos, Theo; Murray, Christopher J L; Forouzanfar, Mohammad H (9 August 2016). " hysical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013". BMJ. 354: i3857. doi:10.1136/bmj.i3857. PMC 4979358 Freely accessible. PMID 27510511. hysical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013". BMJ. 354: i3857. doi:10.1136/bmj.i3857. PMC 4979358 Freely accessible. PMID 27510511.
141. Notterman DA, Mitchell C (2015). "Epigenetics and Understanding the Impact of Social Determinants of Health". Pediatric Clinics of North America (Review). 62 (5): 1227–40. doi:10.1016/j.pcl.2015.05.012. PMC 4555996 Freely accessible. PMID 26318949.
142. Quinlan J, Tu MT, Langlois EV, Kapoor M, Ziegler D, Fahmi H, Zunzunegui MV (2014). " rotocol for a systematic review of the association between chronic stress during the life course and telomere length". Syst Rev (Review). 3 (40): 40. doi:10.1186/2046-4053-3-40. PMC 4022427 Freely accessible. PMID 24886862. rotocol for a systematic review of the association between chronic stress during the life course and telomere length". Syst Rev (Review). 3 (40): 40. doi:10.1186/2046-4053-3-40. PMC 4022427 Freely accessible. PMID 24886862.
143. Noordam R, Gunn DA, Tomlin CC, Rozing MP, Maier AB, Slagboom PE, Westendorp RG, van Heemst D, de Craen AJ, Leiden Longevity Study group (2012). "Cortisol serum levels in familial longevity and perceived age: the Leiden longevity study". Psychoneuroendocrinology. 37 (10): 1669–75. doi:10.1016/j.psyneuen.2012.02.013. PMID 22429748.
144. Lazzarino AI, Hamer M, Gaze D, Collinson P, Steptoe A (2013). "The association between cortisol response to mental stress and high-sensitivity cardiac troponin T plasma concentration in healthy adults". J Am Coll Cardiol. 62 (18): 1694–1701. doi:10.1016/j.jacc.2013.05.070. PMC 3807660 Freely accessible. PMID 23810896.
145. Holt-Lunstad J, Smith TB, Layton JB (2010). "Social Relationships and Mortality Risk: A Meta-analytic Review". PLOS Medicine. 7 (7): e1000316. doi:10.1371/journal.pmed.1000316. PMC 2910600 Freely accessible. PMID 20668659.
146. "Social ties are good for your health - BeWell@Stanford". stanford.edu. Archived from the original on 11 September 2016.
147. Koenig H. G., King D. E., Carson V. B (2012). Handbook of religion and health (2nd ed.). New York: Oxford University Press. p476
148. "Married Vs Single: What Science Says Is Better For Your Health". medicaldaily.com. 2 April 2015.
149. Shor E, Roelfs DJ, Bugyi P, Schwartz JE (2012). "Meta-analysis of marital dissolution and mortality: reevaluating the intersection of gender and age". Soc Sci Med. 75 (1): 46–59. doi:10.1016/j.socscimed.2012.03.010. PMC 3881174 Freely accessible. PMID 22534377.
150. Lam YY, Peterson CM, Ravussin E (2013). "Resveratrol vs. calorie restriction: data from rodents to humans". Exp Gerontol. 48 (10): 1018–24. doi:10.1016/j.exger.2013.04.005. PMID 23624181.
151. Pryor, R; Cabreiro, F (1 November 2015). "Repurposing metformin: an old drug with new tricks in its binding pockets". The Biochemical Journal. 471 (3): 307–22. doi:10.1042/bj20150497. PMC 4613459 Freely accessible. PMID 26475449.
152. Powers RW, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S (2006). "Extension of chronological life span in yeast by decreased TOR pathway signaling". Genes Dev. 20 (2): 174–84. doi:10.1101/gad.1381406. PMC 1356109 Freely accessible. PMID 16418483.
153. Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA (2009). "Rapamycin fed late in life extends lifespan in genetically heterogeneous mice". Nature. 460 (7253): 392–5. Bibcode:2009Natur.460..392H. doi:10.1038/nature08221. PMC 2786175 Freely accessible. PMID 19587680. Lay summary – The Times (8 July 2009).
154. Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R (February 2011). "Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice". J. Gerontol. A Biol. Sci. Med. Sci. 66 (2): 191–201. doi:10.1093/gerona/glq178. PMC 3021372 Freely accessible. PMID 20974732.
155. Ingram DK, Roth GS (March 2011). "Glycolytic inhibition as a strategy for developing calorie restriction mimetics". Experimental Gerontology (review). 46 (2–3): 148–154. doi:10.1016/j.exger.2010.12.001. PMID 21167272.
156. Tardif, S; Ross, C; Bergman, P; Fernandez, E; Javors, M; Salmon, A; Spross, J; Strong, R; Richardson, A (19 July 2014). "Testing Efficacy of Administration of the Antiaging Drug Rapamycin in a Nonhuman Primate, the Common Marmoset". J Gerontol a Biol Sci Med Sci. 70 (5): 577–588. doi:10.1093/gerona/glu101. PMC 4400395 Freely accessible. PMID 25038772.
157. Callaway, Ewen (2010). "Telomerase reverses ageing process". Nature. doi:10.1038/news.2010.635.
158. Blagosklonny, M. V. (2009). "Validation of anti-aging drugs by treating age-related diseases". Aging. 1 (3): 281–8. PMC 2806014 Freely accessible. PMID 20157517.
159. Kogan, Valeria; Molodtcov, Ivan; Menshikov, Leonid I.; Shmookler Reis, Robert J.; Fedichev, Peter (2015). "Stability analysis of a model gene network links aging, stress resistance, and negligible senescence". Scientific Reports. 5: 13589. arXiv:1408.0463 Freely accessible. Bibcode:2015NatSR...513589K. doi:10.1038/srep13589.
160. "Scientists' Open Letter on Aging" Archived 29 April 2015 at the Wayback Machine.. imminst.org.
161. Miller, Richard A.; Harrison, David E.; Astle, Clinton M.; Floyd, Robert A.; Flurkey, Kevin; Hensley, Kenneth L.; Javors, Martin A.; Leeuwenburgh, Christiaan; Nelson, James F.; Ongini, Ennio; Nadon, Nancy L.; Warner, Huber R.; Strong, Randy (2007). "An aging Interventions Testing Program: Study design and interim report". Aging Cell. 6 (4): 565–75. doi:10.1111/j.1474-9726.2007.00311.x. PMID 17578509.
162. "Ageing". Gero. Retrieved 2015-02-04.
163. "Science for Life Extension". Science against aging foundation. Archived from the original on 18 February 2015. Retrieved 2015-02-03.
164. "FAQ – Palo Alto Longevity Prize". Palo Alto Longevity Prize. Retrieved 1 October 2014.
165. Ashlee Vance (9 September 2014). "Silicon Valley Investor Backs $1 Million Prize to End Death". Bloomberg Businessweek. Retrieved 1 October 2014.
166. "$1 Million Longevity Prize Seeks To "Hack The Aging Code"" (Press release). Yahoo! Finance. 9 September 2014. Archived from the original on 6 October 2014. Retrieved 1 October 2014.
167. Aaron Kinney (14 September 2014). "Silicon Valley launches another bid to 'hack' aging, cheat death". San Jose Mercury News. Retrieved 1 October 2014.
168. Victoria Thorp (23 November 2014). "The Palo Alto Prize: A 'Moonshot' at Increasing Longevity". Palo Alto Pulse. Retrieved 8 December 2014.
169. Phillips, Judith, Kristine Ajrouch, and Sarah Hillcoat-Nallétamby (2010) Key Concepts in Social Gerontology. SAGE Publications. ISBN 978-1-4462-0428-3. pp. 12–13.
170. "Ageing in the Twenty-First Century". UNFPA. 2012.
171. LO, W. (2015). The music culture of older adults in Cantonese operatic singing lessons. Ageing and Society, 35(8), 1614-1634. doi:10.1017/S0144686X14000439
172. Vincent, John A. (2005). "Understanding generations: Political economy and culture in an ageing society". The British Journal of Sociology. 56 (4): 579–99. doi:10.1111/j.1468-4446.2005.00084.x. PMID 16309437.
173. "Population Ageing and Development". UNFPA. 2002.
174. "Ageing - UNFPA - United Nations Population Fund". unfpa.org.
175. "UN Human Development Report 2005" (PDF). United Nations Development Programme. Archived from the original (PDF) on 27 May 2008. Retrieved 7 October 2010.
176. Chosewood, L. Casey. "Safer and Healthier at Any Age: Strategies for an Aging Workforce". NIOSH Science Blog. National Institute for Occupational Safety and Health. Retrieved 6 August 2012.
177. Basakha M; Yavari K; Sadeghi H; Naseri A. (2015). "Population Aging And Iran's Non-Oil Economic Growth". Payavard Salamat. 9 (2): 131–146.
178. Basakha M, Yavari K, Sadeghi H, Naseri A (2014). "Ageing and Macroeconomics; Healthcare cost disease as a threat to Iranian ageing society". Journal of Research in Health Sciences. 14 (2): 152–6. PMID 24728752.
179. Scheid, Teresa L.; Brown, Tony N. (2010). A Handbook for the Study of Mental Health (Second ed.). New York: Cambridge University Press.
180. Panek, Paul E.; Hayslip, Bert (1989). Adult development and aging. San Francisco: Harper & Row. ISBN 0-06-045012-6.[page needed]
181. Anita Wejbrandt (2014) Defining aging in cyborgs: A bio-techno-social definition of aging, Journal of Aging Studies, Volume 31, Pages 104–109
182. Chawla, Mukesh; Dubois, Hans F. W.; Chawla, Richard B. (2006). "The Impact of Aging on Long-Term Care in Europe and Some Potential Policy Responses". International Journal of Health Services. 36 (4): 719–46. doi:10.2190/AUL1-4LAM-4VNB-3YH0. PMID 17175843.
183. Reinhardt, U. E. (2003). "Does the Aging of the Population Really Drive the Demand for Health Care?". Health Affairs. 22 (6): 27–39. doi:10.1377/hlthaff.22.6.27. PMID 14649430.
184. Meara, E.; White, C.; Cutler, D. M. (2004). "Trends in Medical Spending by Age, 1963–2000". Health Affairs. 23 (4): 176–83. doi:10.1377/hlthaff.23.4.176. PMID 15318578.
185. Idler, E. L. (2003). "Discussion: Gender Differences in Self-Rated Health, in Mortality, and in the Relationship Between the Two". The Gerontologist. 43 (3): 372–375. doi:10.1093/geront/43.3.372.
186. Deeg, D. J. H.; Bath, P. A. (2003). "Self-Rated Health, Gender, and Mortality in Older Persons: Introduction to a Special Section". The Gerontologist. 43 (3): 369–71. doi:10.1093/geront/43.3.369. PMID 12810900.
187. Benyamini, Y.; Blumstein, T.; Lusky, A.; Modan, B. (2003). "Gender Differences in the Self-Rated Health-Mortality Association: Is It Poor Self-Rated Health That Predicts Mortality or Excellent Self-Rated Health That Predicts Survival?". The Gerontologist. 43 (3): 396–405; discussion 372–5. doi:10.1093/geront/43.3.396. PMID 12810904.
188. Kunzmann, Ute; Little, Todd D; Smith, Jacqui (2000). "Is age-related stability of subjective well-being a paradox? Cross-sectional and longitudional evidence from the Berlin Aging Study". Psychology and Aging. 15 (3): 511–526. doi:10.1037/0882-7974.15.3.511.
189. Jylhä, Marja; Guralnik, Jack M; Balfour, Jennifer; Fried, Linda P (2001). "Walking Difficulty, Walking Speed, and Age as Predictors of Self-Rated Health: The Women's Health and Aging Study". Journal of Gerontology. 56A (10): M609–17. PMID 11584033.
190. Heckhausen, Jutta (1999). Developmental Regulation in Adulthood: Age-Normative and Sociostructural Constraints as Adaptive Challenges. Cambridge University Press. ISBN 978-0-521-02713-7.
191. Sargent-Cox, Kerry; Anstey, Kaarin; Luszcz, Mary (2008). "Determinants of Self-Rated Health Items With Different Points of Reference". Journal of Aging and Health. 20 (6): 739–761. doi:10.1177/0898264308321035.
192. Idler, Ellen L (1993). "Age differences in self-assessments of health: Age changes, cohort difference, or survivorship?". Journal of Gerontology. 48 (6): S289. doi:10.1093/geronj/48.6.s289.
193. Williamson, JD; Fried, LP (1996). "Characterization of older adults who attribute functional decrements to "old age"". Journal of the American Geriatrics Society. 44 (12): 1429–1434. doi:10.1111/j.1532-5415.1996.tb04066.x.
194. Baltes, Paul B.; Baltes, Margret M. (1990). "Psychological perspectives on successful aging: The model of selective optimization with compensation". In Baltes, Paul B.; Baltes, Margret M. Successful Aging. pp. 1–34. doi:10.1017/CBO9780511665684.003. ISBN 978-0-511-66568-4.
195. Rowe, J.; Kahn, R. (1987). "Human aging: Usual and successful". Science. 237 (4811): 143–9. Bibcode:1987Sci...237..143R. doi:10.1126/science.3299702. PMID 3299702.
196. Job 14:5-7 A man’s days are numbered. You know the number of his months. He cannot live longer than the time You have set. So now look away from him that he may rest, until he has lived the time set for him like a man paid to work. For there is hope for a tree, when it is cut down, that it will grow again, and that its branches will not stop growing |